Mitchell and Ben
Period 6, 2/19/10
I. Title: Polarity and Molecular Shape Lab
II. Statement of the Problem:
a. How shape affects polarity.
b. How elements combine to form molecules.
III. Hypothesis:
a. We hypothesize that based on the shape of a molecule we can determine whether or not the
molecule is polar.
IV. Materials:
a. Ball and stick model
 sets, pencils, paper, molecular shape chart (see fig. 1), and a camera.
sets, pencils, paper, molecular shape chart (see fig. 1), and a camera.fig. 1
V. Procedure:
a. We drew a Lewis structure based on the chemical formula.
b. Using the shape chart we determined the actual molecular shape.
c. We then made a model based on the actual molecular shape and the Lewis structure that we made.
d. Then we proceeded to determine if the molecule could be polar based on the shape of the molecule.
e. We then drew the model and took several pictures of the models.
VI. Results:
a. We took the following pictures.


Water: H2O
(Polar)


C2H4


C3 H8


BF3

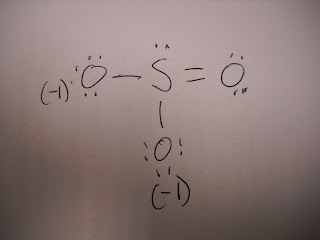 SO3-2
SO3-2(Resonance Structure)
VII. Conclusions:
a. We determined that it is not only the difference in electronegativity difference of the atoms in a compound that makes a compound polar, it is also the shape as is the case with H2O
b. A compound must have two distinctive halves so that one can be positive and the other can be negative.
c. We also determined the angle's between bonding groups and the shapes of all the molecules.
d. We learned that fluorine can cause molecule to have weird shapes due to it's high
electronegativity.

